Home /
Expert Answers /
Chemistry /
using-the-values-in-the-table-of-naoh-and-the-strong-diproic-acid-ha-at-equivalence-point-eq-cal-pa747
(Solved): Using the values in the table of NaOH and the strong diproic acid HA at equivalence point (EQ), cal ...
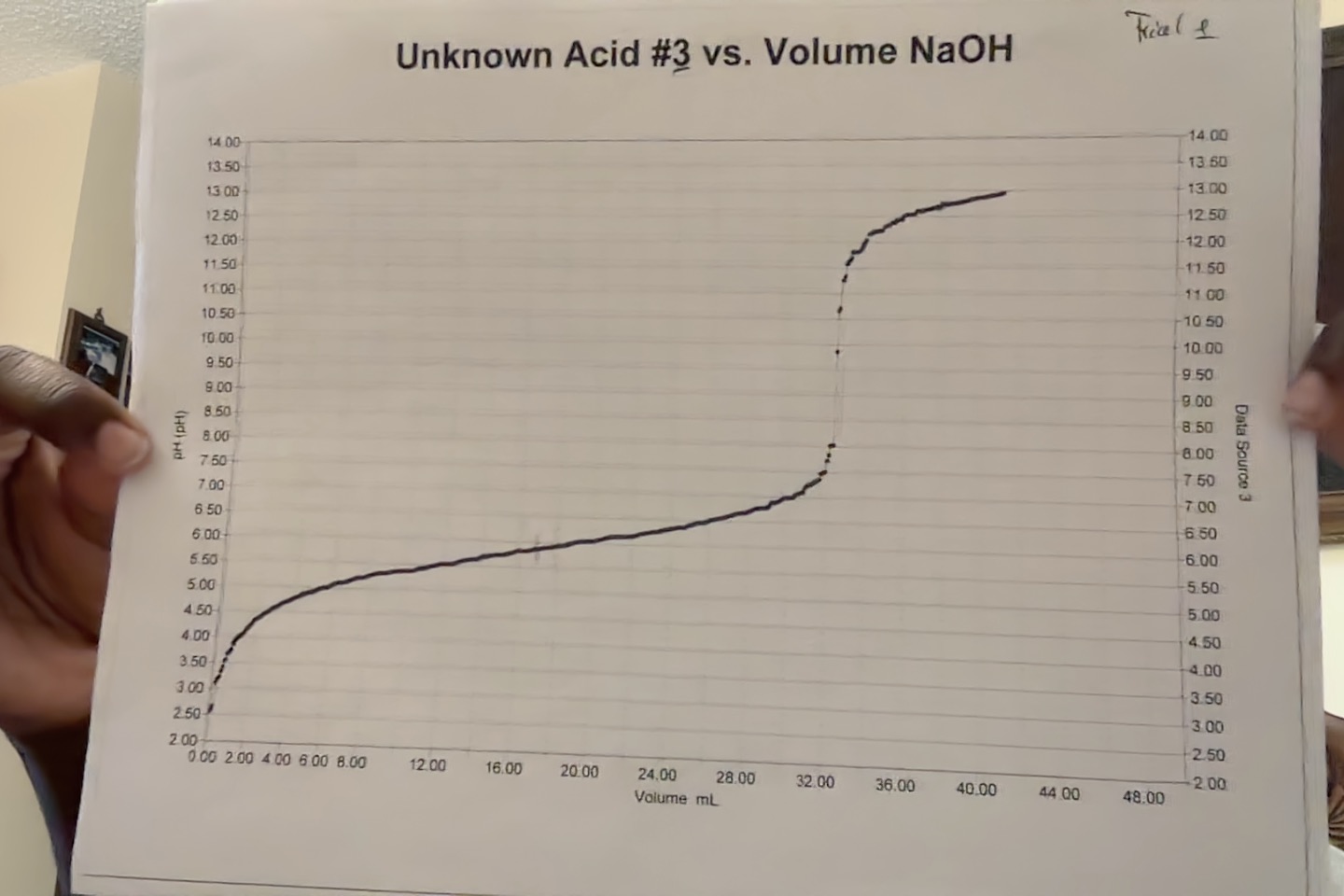
Using the values in the table of NaOH and the strong diproic acid HA at equivalence point (EQ), calculate the pH of their reaction at 1/2 the equivalence point. Once pH found, calculate the pka and ka value.Unknown Acid #3 vs. Volume
NaOH. This is all the information I have. The acid is Hydroxylamine Hydrochloride