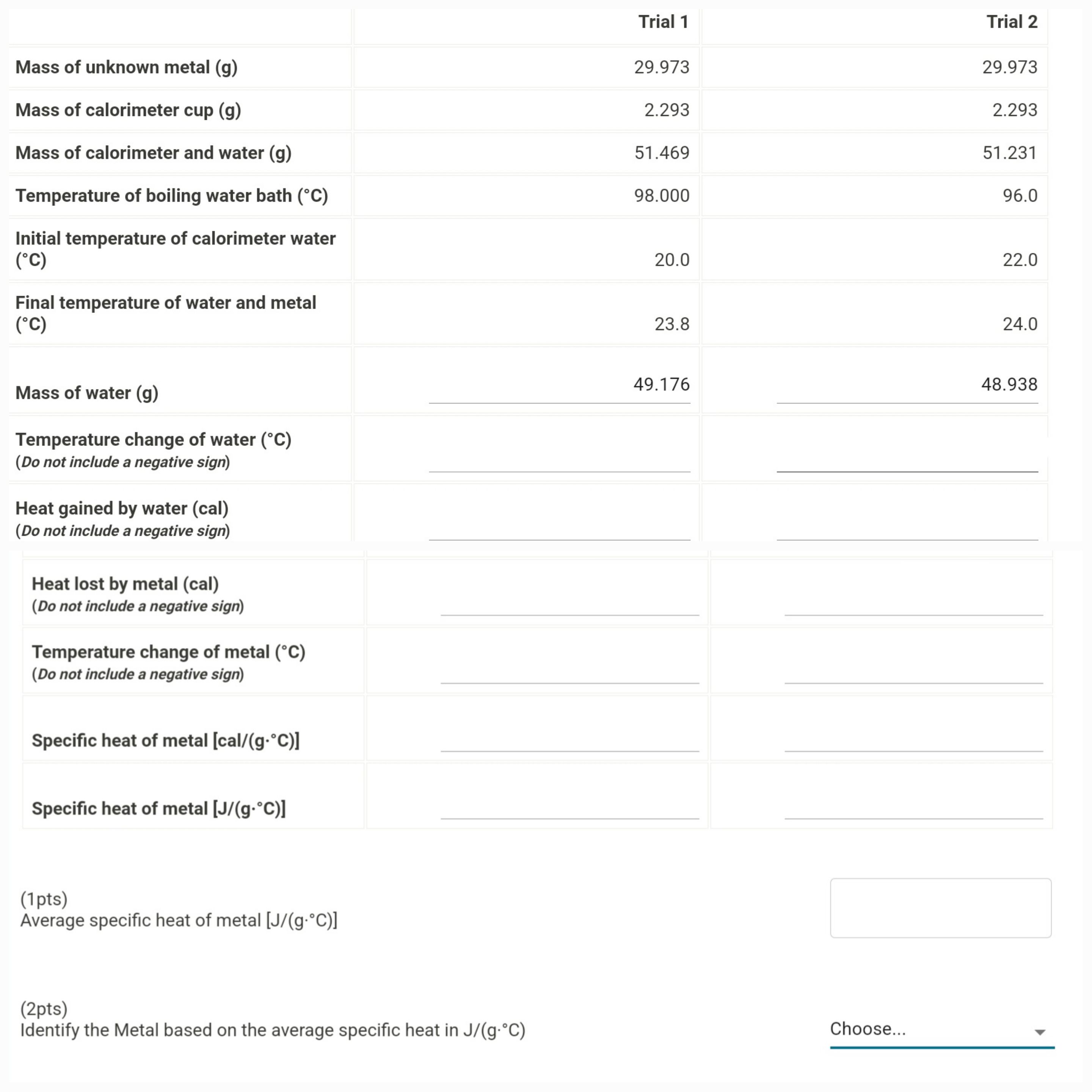
(Solved): What is the correct answer based on the data provided? Calculations for the Specific Heat for an Un ...
What is the correct answer based on the data provided? Calculations for the Specific Heat for an Unknown Metal \table[[,Trial 1,Trial 2],[Mass of unknown metal (g),29.973,29.973],[Mass of calorimeter cup (g),2.293,2.293],[Mass of calorimeter and water (g),51.469,51.231],[Temperature of boiling water bath (\deg C),98.000,96.0],[\table[[Initial temperature of calorimeter water],[(\deg C)]],20.0,22.0],[\table[[Final temperature of water and metal],[(\deg C)]],23.8,24.0],[Mass of water (g),49.176,48.938],[\table[[Temperature change of water ( {:\deg C)],[(Do not include a negative sign)]],78.0,74.0],[\table[[Heat gained by water (cal)],[(Do not include a negative sign)]],,],[\table[[Heat lost by metal (cal)],[(Do not include a negative sign)]],,]]