Home /
Expert Answers /
Chemistry /
what-is-the-standard-cell-potential-of-the-above-cell-o-a-1-10v-o-b-0-430-v-using-the-formu-pa554
(Solved): What is the standard cell potential of the above cell? o A. ,-1.10V O B. -0.430 V ) Using the formu ...
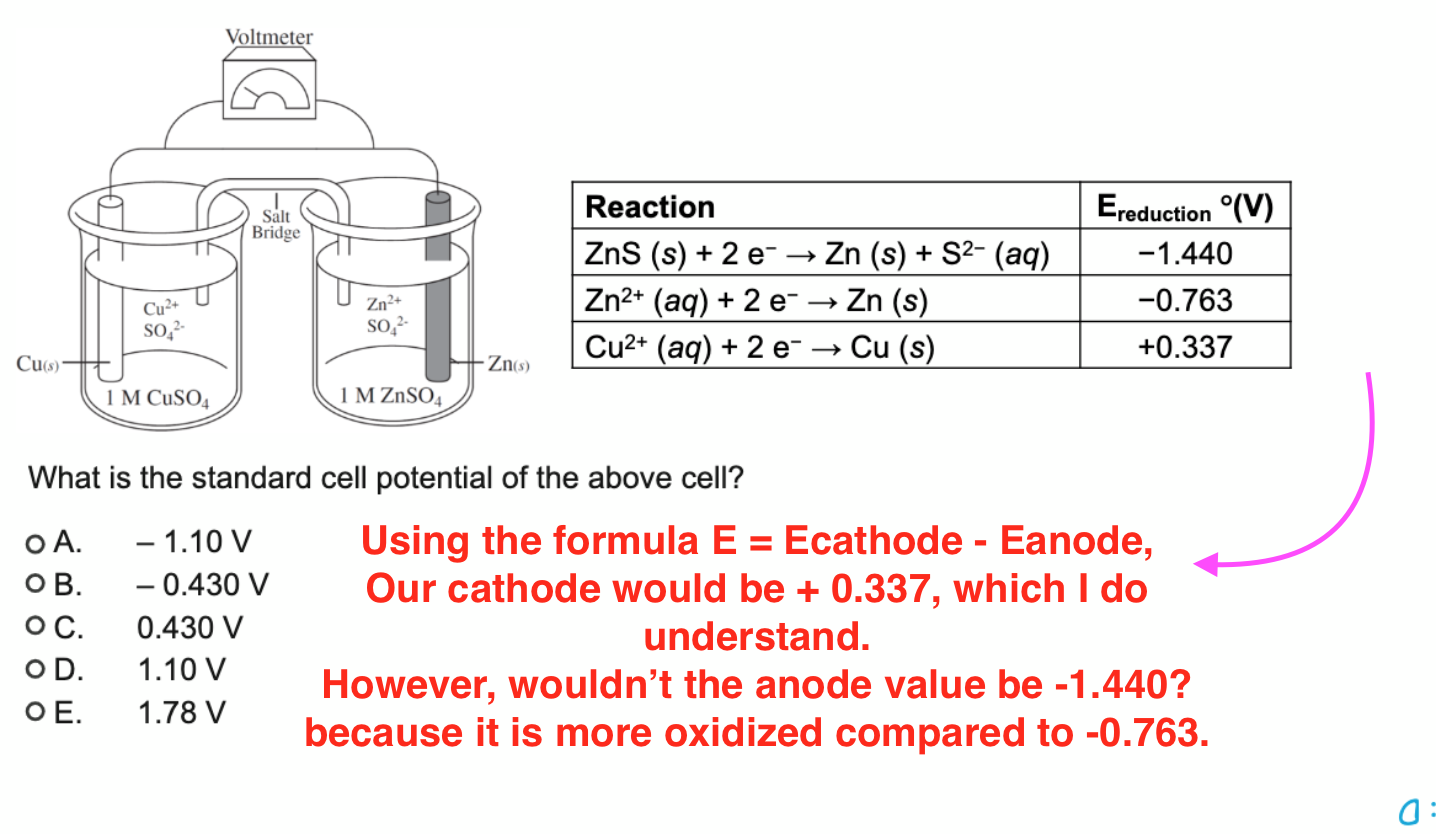
What is the standard cell potential of the above cell? o A.
,-1.10VO B. -0.430 V ) Using the formula
E=Ecathode - Eanode, O C. 0.430 V Our cathode would be +0.337 , which I do O D.
,1.10Vunderstand. O E.
,1.78VHowever, wouldn't the anode value be
-1.440?because it is more oxidized compared to
-0.763.