Home /
Expert Answers /
Chemistry /
which-of-the-following-acids-is-the-best-acid-to-prepare-a-buffer-with-the-highest-ph-c-5-h-5-o-pa258
(Solved): Which of the following acids is the best acid to prepare a buffer with the highest pH ? C_(5)H_(5)O_ ...
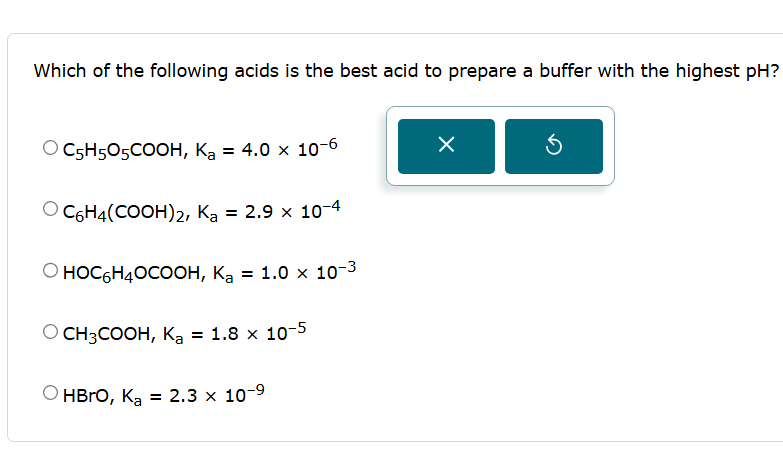
Which of the following acids is the best acid to prepare a buffer with the highest pH ?
C_(5)H_(5)O_(5)(C)/(O)O H,K_(a)=4.0\times 10^(-6)
C_(6)H_(4)((C)/(O)O H)_(2),K_(a)=2.9\times 10^(-4)
HOC_(6)H_(4)O(C)/(O)O H,K_(a)=1.0\times 10^(-3)
CH_(3)(C)/(O)O H,K_(a)=1.8\times 10^(-5)
HBrO, K_(a)=2.3\times 10^(-9)