Home /
Expert Answers /
Chemistry /
which-of-the-following-are-aromatic-molecules-if-they-are-aromatic-how-many-unique-aromatic-rings-pa166
(Solved): Which of the following are aromatic molecules. If they are aromatic, how many unique aromatic rings ...
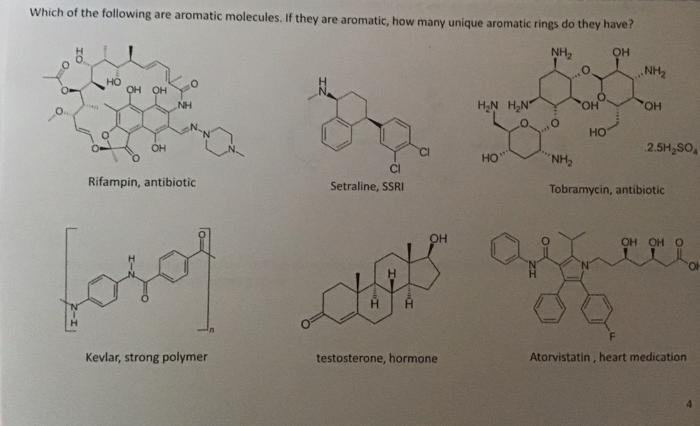
Which of the following are aromatic molecules. If they are aromatic, how many unique aromatic rings do they have? Rifampin, antibiotic Setraline, SSRI Tobramycin, antibiotic Kevlar, strong polymer testosterone, hormone Atorvistatin, heart medication
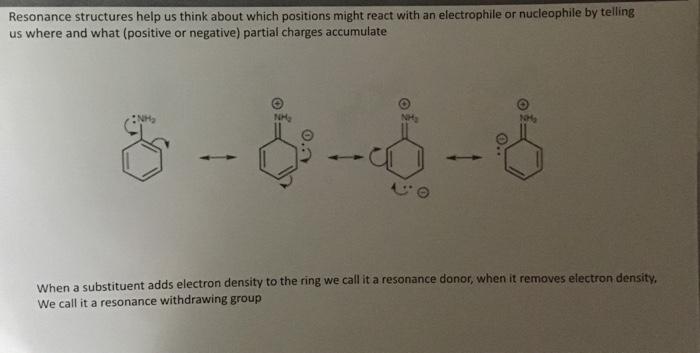
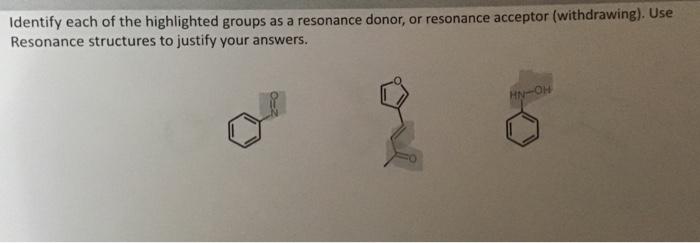
Resonance structures help us think about which positions might react with an electrophile or nucleophile by telling us where and what (positive or negative) partial charges accumulate When a substituent adds electron density to the ring we call it a resonance donor, when it removes electron density. We call it a resonance withdrawing group
\&
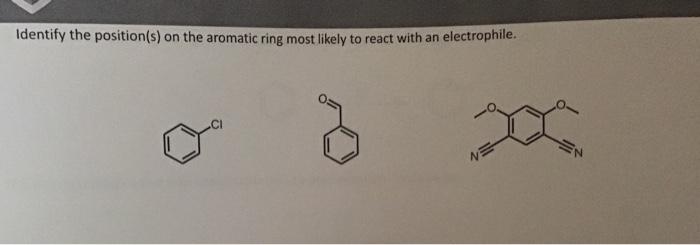
Identify the position(s) on the aromatic ring most likely to react with an electrophile.
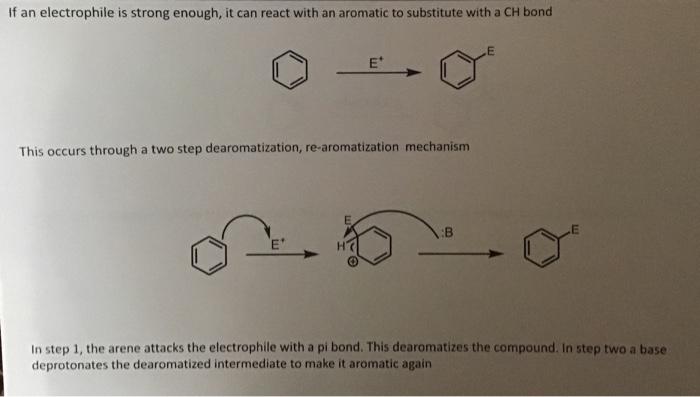
If an electrophile is strong enough, it can react with an aromatic to substitute with a bond This occurs through a two step dearomatization, re-aromatization mechanism In step 1, the arene attacks the electrophile with a pi bond. This dearomatizes the compound. In step two a base deprotonates the dearomatized intermediate to make it aromatic again
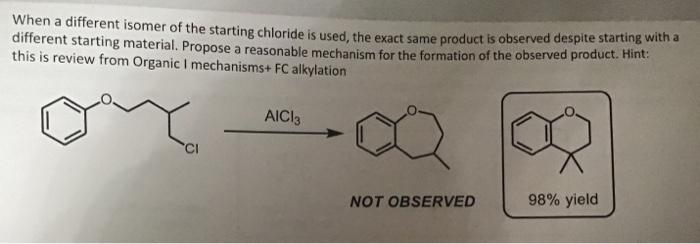
When a different isomer of the starting chloride is used, the exact same product is observed despite starting with a different starting material. Propose a reasonable mechanism for the formation of the observed product. Hint: this is review from Organic I mechanisms alkylation