Home /
Expert Answers /
Chemistry /
write-the-cell-notation-for-an-electrochemical-cell-consisting-of-an-anode-where-mathbf-c-u-pa162
(Solved): Write the cell notation for an electrochemical cell consisting of an anode where \( \mathbf{C u}(\ ...
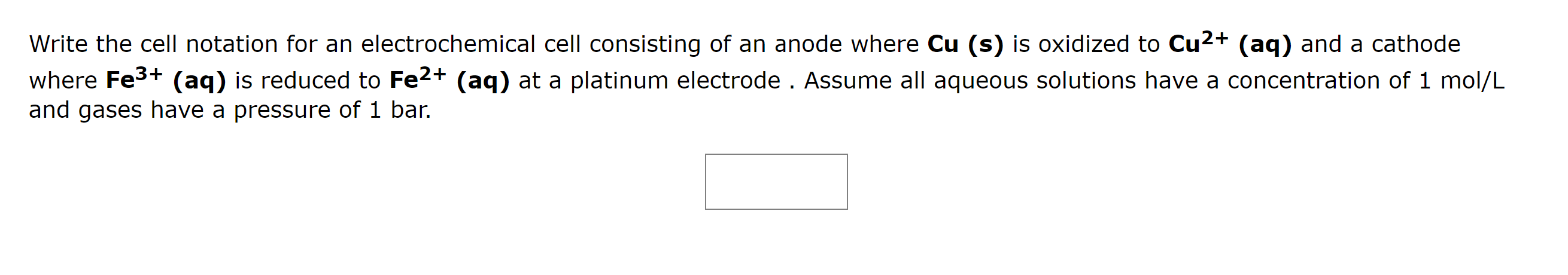
Write the cell notation for an electrochemical cell consisting of an anode where \( \mathbf{C u}(\mathbf{s}) \) is oxidized to \( \mathbf{C u}^{\mathbf{2 +}} \mathbf{( a q )} \) and a cathode where \( \mathbf{F e}^{3+}(\mathbf{a q}) \) is reduced to \( \mathbf{F e}^{2+}(\mathbf{a q}) \) at a platinum electrode. Assume all aqueous solutions have a concentration of 1 mol/L and gases have a pressure of 1 bar.
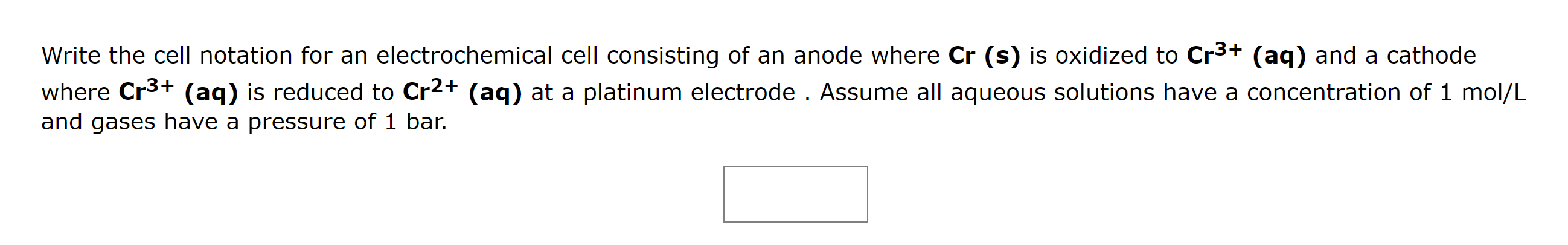
Write the cell notation for an electrochemical cell consisting of an anode where \( \mathbf{C r}(\mathbf{s}) \) is oxidized to \( \mathbf{C r}^{3+}(\mathbf{a q}) \) and a cathode where \( \mathbf{C r}^{3+} \mathbf{( a q )} \) is reduced to \( \mathbf{C r}^{2+}(\mathbf{a q}) \) at a platinum electrode. Assume all aqueous solutions have a concentration of 1 mol/L and gases have a pressure of 1 bar.